Notes From the Upside-Down: Lipid Nano Particles, Lies of Omission on Wikipedia, by German MSM, and the EU Commission Show: Danger!
I went down further that particular rabbit hole--we're flying blind, the EU Commission is lying through their teeth, and Wikipedia is, well, 'compromised': what could go wrong…?!
I really didn’t want to do this post, because it’s yet another one showing the close, if not outright incestuous collaboration—of conspiracy—between Big Pharma, the Deep State, and German legacy media when it comes to all things Covid-19. But then again, when I told my wife about it, she complained that no one is doing so, well, here goes.
Q: what is ALC-0315 (and SM-102)?
Please refer to both Jessica Rose’s piece (on SM-102) and yesterday’s article on ALC-0315 for details. In all brevity, both are pharmaceutical research products that play an important role in the mRNA injections currently available (pushed) against Sars-Cov-2 and Covid-19. They are key components of the lipid nanoparticles (LNP) that allow the synthetic mRNA to enter a ‘vaccinated’ person’s cells. In other words, without these products, neither the BioNTech/Pfizer nor the Moderna injections would work.
Q: ok, what’s the rub?
While it sounds kinda ‘cool’, note that both products are labelled explicitly ‘not for human use’ and ‘research only’, as they are both quite toxic.
Q: hmmm, do we know how toxic? I mean, what kind of toxicity are we talking about?
We don’t know.
No regulatory authority that has invested both products with Emergency Use Authorisation (EUA) has required the manufacturers—BioNTech/Pfizer, Moderna—to actually carry about genotoxicity or carcinogenicity studies.
In other words: governments and public health authorities knew about these components when they granted the EUAs—by which is meant ‘conditional marketing authorisation’—to both BioNTech/Pfizer and Moderna. As no studies were required about the potential of either injection to lead to cancer and/or damages to one’s genetic information, the past 15 or so months (and counting) cannot but be considered the single largest medical experiment ever conducted.
Q: that sounds…bad. Why isn’t this talked about more by, say, governments, regulators, or medical professionals?
Basically, a lot of people do talk about it, from world-leading medical experts such as Peter McCoullough, Pierre Kory, and Paul Marik to more research-focused individuals like Robert W. Malone, Paul Alexander, Jessica Rose—but their voices are all but suppressed by the powerful collusion between governments, public health authorities, and big tech, all in apparent cahoots with the Phascists over at BioNTech/Pfizer, Moderna, and their ilk.
If you touch this topic as a journalists—and there are only a few of these people who remain—it’s almost as if you’ve broken the omertà, the Mafia’s vow of silence (watch The Godfather trilogy). If you like, follow Alex Berenson’s substack-related suit against guano island.
Q: alright, you wanted to bring up something about this, so, any ‘infamous’ one-liners here to sum this up?
To me, the above collusion between governments, public health authorities, and big tech resembles a stereotypical teenager who’s been caught doing something really stupid, with all the evidence lying cluttered around him or her but who, at first, insists that ‘it wasn’t me’.
Q: well, we all know what happens after that moment, don’t we?
Huhum, I suspect that, as spring turns to summer, with inflation (currently around 7-8% yoy) ramping up, governments openly discussing the ‘benefits’ of a second ‘booster’ (4th dose, if you’re counting) vs. the (re-) introduction of mandates—if Johnny Q. Public won’t take another shot—we’ll see the narrative shift from ‘it wasn’t me’ to ‘look mom and dad, I didn’t know this would happen’.
At that point, we’ll find learn if the Judiciary is an actually independent branch of government. If yes, there will be the mother of all legal proceedings, with indictments and long-term prison sentences meted out against all those who knowingly harmed the public; in this context, why not bring back the death penalty for high treason (in those countries that abolished it), for if you think this through, I don’t know if anything before—from the Dreyfuß affair to the witchhunts of the McCarthy era and beyond—wouldn’t actually pale in comparison.
If not (which is what I think will happen), you will see a closing of the ranks of those in power, which should reveal, to large segments of the population, that we’ve all been had: we’ve believed in the fiction of accountability and, above all, constitutional principles enshrined in law. Talk about Soviet-era Eastern Europe ‘going global’.
Q: that sounds…appalling. Do you have any evidence to back up your claims?
As the header of this post implies, I think I do. Please follow me down this particular rabbit hole, and for all practical purposes, we’ll start with…Wikipedia on ALC-0315.
Wikipedia, ‘Truth’ and Fiction
There are, of course, Wikipedia entries for ALC-0315 in both English and German (as well as in a bunch of other languages). Let’s briefly focus on the German version, though, which differs quite substantially from its English ‘peer’:
As the picture shows, there are two major differences between these two entries: in the first paragraph, the German version holds that ALC-0315 is ‘the main ingredient in the lipid admixture in…BNT162b2’ (Es bildet die Hauptkomponente des Lipidgemisches im SARS-CoV-2-Impfstoff BNT162b2).
Yet, as highlighted by the big yellow arrow, there’s another element that is different from the English entry: the German version boasts a ‘security label’ (Sicherheitshinweis) according to the Globally Harmonized System of Classification, Labelling and Packaging of Chemicals, or GHS, which for ALC-0315 holds:
No classification available [keine Einstufung verfügbar]
Well, there’s a footnote (1), which reads as follows:
(1) This substance has either not yet been classified in terms of its hazard or a reliable and citable source on this has not yet been found.
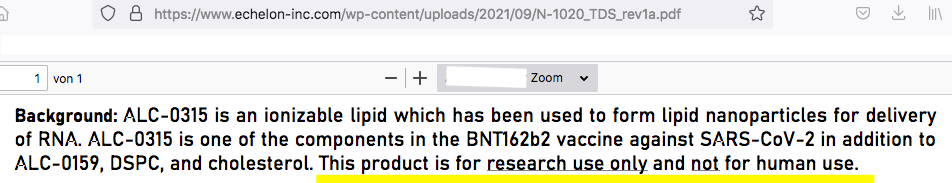
As a public service announcement, here’s some information on this substance, which comes from its manufacturer, Echelon Biosciences Inc.
So far, So Good—On to the English Entry
Interestingly enough, the English Wikipedia entry looks a bit different:
There is no mention of the safety concerns in the overview box on the right, but there are a few curious lines in the ‘Use’ section that sparked my interest. From the above entry, and I quote (links omitted):
In December 2021 there were objections raised against the use of ALC-0315 and ALC-0159 (and some other solid lipid nanoparticles) in humans by critics of mRNA-COVID-19 vaccines in Germany but the German pharmaceutical trade journal Pharmazeutische Zeitung (de) and the German investigative collective Correctiv refuted this and stated as one important reason that the European Medicines Agency has approved the vaccine and this includes all its ingredients.[9][10]
Shall we now look at the footnotes?
References (9) and (10) lead to two German-language pieces on ALC-0315 that appeared earlier this year, which I then checked out.
German Media Writes About This (But It’s Unknown to German Wikipedia)
Annette Rößler, writing in the Pharmazeutische Zeitung (Pharmaceutical Journal, the German Pharmacists’ trade journal), published the following on 18 Jan. 2022:

A bit further down (second paragraph, all but one reference omitted, my emphasis), there is this:
The lipid nanoparticles in Comirnaty are composed of the components DSPC, ALC-0315 and ALC-0159, and cholesterol, with DSPC and cholesterol being the main components. ALC-0315 and ALC-0159 are two novel lipids, which means they have not been included in any approved drug or vaccine before. The same is true for the two lipids SM-102 and PEG2000-DMG, which are excipients in Moderna's Spikevax®. Since Comirnaty and Spikevax are the first mRNA vaccines to be approved, this is not surprising: these lipids have very specific properties that were not previously required.
I’ve included only one link in this paragraph, which leads to (an undated) piece on ‘The Nanotechnology of Covid-19 Vaccines’ (note that the piece reads ‘Vakzine’ in German, which is a rather recent terminological import into the German language that differs from the traditional term, ‘Impfung’), which has the following to say about ALC-0315:
The functional lipid ALC-0315 is a novel tertiary amine that is uncharged at physiological pH. During particle preparation under acidic pH conditions, it serves to bind the polyanionic mRNA. After application of the LNP and its cellular uptake into the endosomal cell compartment, it imparts a positive charge to the nanoparticles, which ultimately leads to translocation of the mRNA into the cytosol of the cell. Thus, this lipid is essentially responsible for successful drug application…
The two functional lipids ALC-0315 and ALC-0159 are novel excipients that have not yet been found in approved finished medicinal products.
Is it fair now to call Ms. Rößler’s above characterisation as ‘disingenuous’? By the way, she also contradicts the German Wikipedia entry that holds that ALC-0315 is actually ‘the main ingredient in the lipid admixture in…BNT162b2’.
One last thing to note: in that second piece from the Pharmazeutische Zeitung the concluding paragraph reads as follows (my emphases):
Pharmacokinetically, the vaccine is characterised by the fact that the highest concentrations are found at the injection site, but a redistribution is observed over time. An accumulation in the liver is typical for nanoparticulate dosage forms and also described for LNP. In pharmacokinetic studies with radiolabelled vaccine, up to 21.5 per cent of the injected dose was detected in the liver and significantly lower amounts in the spleen, adrenal glands and ovaries.
Back to Ms. Rößler’s Piece
To her credit, there are some factually correct statements in Ms. Rößler’s piece (my emphases):
As reported by the website Correctiv, among others, since mid-December [2021] the claim has been spreading on vaccine-critical websites and social networks that the adjuvants ALC-0315 and ALC-0159 are not approved for use in humans. On 22 December, AfD [Alternative für Deutschland] MEP Guido Reil submitted a parliamentary enquiry to the EU Commission to this effect. The lipids ALC-0315 and ALC-0159 used in Comirnaty are produced by the US company Echelon Biosciences and are, according to their information, ‘for research only and not for human use’. The additives are therefore illegal, and the use of the vaccine is ‘illegal, dangerous and unethical’.
You see, Ms. Rößler managed to ‘perform’ journalism here by mentioning, correctly, the manufacturer of ALC-0315 but without citing any direct source. Ms. Rößler continues as follows, showing off her awesome abilities of illogical reasoning™:
Yet, this is incorrect for several reasons. Firstly, it is not true that the additives are unauthorised. This is because Comirnaty is approved and therefore all the additives it contains are also approved.
You see, there’s no need to differentiate between an EUA (‘conditional marketing authorisation’) and the full licensure by a regulatory agency. Back to Ms. Rößler:
Secondly, it is not true that Echelon is the manufacturer of the adjuvants used in Comirnaty—and this is important because the Echelon product information on ALC-0315 and ALC-0159 actually contains the cited clause [i.e., ‘for research use only and not for human use’]. In a statement published on its website in the meantime, however, the company also explains how this is to be understood: the reference is important, it says, because in the case of substances used for research purposes, the requirements for manufacture are less stringent than in the case of intended use in humans.
Here’s the statement by Echelon, by the way:
Do you feel safe(r) now?
If not, here’s what the Paul Ehrlich Institute has to say.
These claims are buttressed by something else Ms. Rößler cites, which is a Q&A from the Paul Ehrlich Institute, Germany’s pharmacological watchdog, released on 23 Dec. 2021:
Is it true that Comirnaty (BioNTech/Pfizer) and Spikevax (Moderna) use ingredients that are not allowed in medicinal products?
No.
The substances ALC-0315 and ALC-0159 in the vaccine Comirnaty (BioNTech/Pfizer) and SM-102 in the vaccine Spikevax (Moderna) are pharmaceutical adjuvants. Pharmaceutical adjuvants can be produced by the drug manufacturer itself or purchased from corresponding companies. Such substances are sometimes offered as laboratory chemicals for a wide variety of applications. The manufacturer usually provides the product information on these laboratory chemicals with a warning that they are not suitable for use in humans. This can lead to the erroneous assumption that they generally cannot be used in humans.
As soon as such substances are used in medicinal products, their suitability for use in humans must be carefully examined and evaluated by the manufacturer and within the framework of the marketing authorisation, e.g. by the Paul Ehrlich Institute. A marketing authorisation application contains corresponding information on quality and production. The above-mentioned testing was also carried out as usual for the approval of mRNA vaccines.
What about now?
Finally, here’s the EU Commission’s answer to MEP Guido Reil
Mr. Reil had asked the following on 22 Dec. 2021 (my emphasis):
According to the product information supplied by the European Medicines Agency, two of the main components of Pfizer’s Comirnaty vaccine are ALC-0315 and ALC-0159. Echelon, the manufacturer of these nanoparticles, specifies that they are ‘for research only and not for human use’. Administering a vaccine—particularly to children—which contains unauthorised excipients is illegal, dangerous and unethical.
1. How does the Commission justify distributing a product that is harmful to public health and, as such, infringes Article 168(1) of the Treaty on the Functioning of the European Union?
2. How can it explain such a serious oversight—particularly given that the EU founded a European Health Emergency Preparedness and Response Authority (HERA) in September 2021—and how will it avoid similar occurrences in future?
3. What does it intend to do to put an end to the persistent threat that unauthorised vaccine components pose to people in Europe?
On 9 Feb. 2022, Ms. Kyriakides, the EU Commissioner for Health etc., responded as follows (my emphases):
The Commission grants a marketing authorisation to a medicinal product based on the objective scientific criteria of quality, safety and efficacy of the medicinal product concerned, following a recommendation from the European Medicines Agency (EMA).
At any time, if new evidence becomes available, EMA assesses it and, if appropriate, recommends the Commission to amend or suspend the authorisation. Comirnaty was granted a conditional marketing authorisation in December 2020, according to this procedure.
A very significant part of the data that any manufacturer of medicines must supply to regulators relates to the quality of the product and its individual ingredients, as well as to showing the necessary manufacturing processes and controls are in place to maintain the quality in every batch of the medicine.
This means that the excipients used in a medicinal product are scrutinised by the regulatory authorities and are part of the authorised composition.
The quality of the ALC-0159 used as an excipient in Comirnaty has been demonstrated to be appropriate for its intended use and is in compliance with the relevant EMA scientific guidelines and standards expected for all medicines.
Details of EMA’s quality and safety assessment of Comirnaty are available in its public assessment report (1).
Did you notice Ms. Kyriakides’ sleight-of-hand here?
MEP Reil had asked specifically about both ALC-0159 and ALC-0315, yet Ms. Kyriakides referred only to the former.
To her credit, someone working for the EU Commission added a footnote to her answer, which leads to the EMA’s Assessment Report, dated 19 Feb. 2021, which you can find here.
The EMA’s Assessment Report from Feb. 2021
I’m quoting liberally from that piece, all emphases are mine (if not noted otherwise), please refer to the pp. range given to read up on this yourself.
Other ingredients are: ALC-0315 (4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), ALC-0159 (2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide), 1,2- Distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, potassium chloride, potassium dihydrogen phosphate, sodium chloride, disodium phosphate dihydrate, sucrose and water for injections. (pp. 14-15)
All excipients except the functional lipids ALC-0315 and ALC-0159 and the structural lipid DSPC comply with Ph. Eur. The functional lipid excipients ALC-0315 and ALC-0159, are classified as novel excipients…
Two novel excipients are included in the finished product, the cationic lipid ALC-0315 and the PEGylated lipid ALC-0159. Limited information regarding the novel excipients are provided (p. 23)
There’s an entire section on these two lipids (pp. 23-25), from which the below information is taken:
The proposed specification is considered acceptable based on the available data. However, additional information regarding specifications that should be provided (SO4 [SO is EUrocrat-speak for ‘Specific Objectives’, often used in grant applications and tasks set out by EU institutions]).
Stability data from one supplier indicate that ALC-0315 is stable when stored at the recommended storage conditions. Additionally, the excipient is stable at room temperature suitable for use in further manufacturing steps. Stability data from one supplier is considered representative for lipid from another supplier.
Lipid related impurities have been observed in some recently manufactured finished product batches, correlated with ALC-0315 lipid batches. The quality of ALC-0315 excipient is considered acceptable based on the available data on condition that specific impurities in the finished product will be further evaluated (SO2).
In case you’re beginning to have second thoughts after reading these passages, it won’t get better the further down one goes through that piece cited my Commissioner Ms. Kyriakides.
ALC-0315 and ALC-0159 are novel excipients, not previously used in an approved finished product within EU. Additional information is provided separately in Section A.3 of the dossier. (p. 28)
On the ‘Finished product’ (pp. 33-35, quote at 34), we read this:
Two novel excipients are included in the LNP. Complete information is not provided for both the cationic lipid ALC-0315 and the PEGylated lipid ALC-0159. In order to assure comprehensive control throughout the lifecycle of the finished product and to ensure batch to batch consistency, further information needs to be submitted regarding the synthetic process and control strategy in line with specific obligations (SO4, SO5).
On the ‘Specific Objectives’, see the long list on pp. 35-39, from which the below ALC-0315 segments were taken (at 38, Italics in the original, bold emphases mine):
As regards SO4, the data are requested to be provided regarding the synthetic process and control strategy for the excipient ALC-0315 in order to improve the impurity control strategy, assure comprehensive quality control and batch-to-batch consistency throughout the lifecycle of the finished product.
a) A detailed description of the chemical synthesis of ALC-0315 (e.g., information on reagents and process conditions) should be provided. Due date: January 2021…
e) Specified impurities should be further evaluated and appropriate specification limits for individual impurities should be included when more data are available. Acceptance criteria for specified and un-specified impurities should be added to the specification for ALC-0315 and should also be evaluated during stability studies. Due date: July 2021, Interim report: April 2021…
f) Detailed method validation reports for assay, impurities, and residual solvents for ALC-0315 should be provided. Due date: July 2021
Translation: we don’t have all the data necessary, but since this is ‘urgent’, we don’t care about it right now.
On ‘Pharmacokinetics’ (pp. 45-48), we read the following:
The applicant has determined the pharmacokinetics of the two novel LNP excipients ALC-0315 (aminolipid) and ALC-0159 (PEG-lipid) in plasma and liver as well as their elimination and metabolism in rats. Furthermore, the Applicant has studied the biodistribution of the two novel lipids (in rats) and the biodistribution of a LNP-formulated surrogate luciferase RNA in mice (IV), as well as the biodistribution of a [3H]-Labelled Lipid Nanoparticle-mRNA Formulation in rats (IM).
No traditional pharmacokinetic or biodistribution studies have been performed with the vaccine candidate BNT162b2. (p. 45)
It get’s ever more worse (pp. 45-46):
ALC-0315 and ALC-0159 levels in plasma, liver, urine and faeces were analysed by LC-MS/MS at different time-points up to 2-weeks.
ALC-0315 and ALC-0159 were rapidly cleared from plasma during the first 24 hours with an initial t½ of 1.62 and 1.72 h, respectively. 24 hours post-dosing, less than 1% of the maximum plasma concentrations remained. A slower clearance rate was observed after 24 hours with ALC-0315 and ALC-0159 terminal elimination t½ of 139 and 72.7 h, respectively.
Following plasma clearance, the liver appears to be to major organ to which ALC-0315 and ALC-0159 distribute. The applicant has estimated the percent of dose distributed to the liver to be ~60% for ALC-0315 and ~20% for ALC-0159. The observed liver distribution is consistent with the observations from the biodistribution study and the repeat-dose toxicology, both using IM administration.
For ALC-0315 (aminolipid), the maximum detected concentration in the liver (294 μg/g liver) was reached 3 hours after IV injection. ALC-0315 was eliminated slowly from the liver and after 2-weeks the concentration of ALC-0315 was still ~25% of the maximum concentration indicating that ALC-0315 would be eliminated from rat liver in approximately 6-weeks. For ALC-0159 (PEG-lipid), the maximum
detected concentration in the liver (15.2 μg/g liver) was reached 30 minutes following IV injection. ALC-0159, was eliminated from the liver faster than ALC-0315 and after 2-weeks the concentration of ALC-0159 was only ~0,04% of the maximum detected concentration. The applicant was asked to discuss the long half-life of ALC-0315 and its effect, discussion on the comparison with patisiran, as well as the impact on the boosts and post treatment contraception duration. The applicant considered that there were no non-clinical safety issues based on the repeat dose toxicity studies at doses (on a mg/kg basis) much greater than administered to humans; this was acceptable to the CHMP.
See, no data beyond two weeks. ALC-0315 and ALC-0159 accumulate in the liver—did anyone check how they interact with, say, Remdesivir? And, when asked about ‘the long half-life of ALC-0315’ (which is never mentioned, by the way), ‘the applicant considered that there were no non-clinical safety issues’ whatever is done and irrespective of repeat administration of their product.
Apparently, ‘considerations’ are enough, like, I’m considering buying a new lamp for the kitchen. What’s safe for the goose, is good enough for the gander, ain’t it?
Both patisaran lipids showed an essentially similar PK profile in clinic with a strongly biphasic profile and long terminal half-lives. According to the applicant, it is difficult to further contextualize the pharmacokinetic data and therefore to understand the safety of these molecules, beyond consideration of dose. There is a large dose differential between the human BNT162b2 dose and the dose used in the toxicity studies (300-1000x) which provides an acceptable safety margin.
Moreover, according to the Applicant given the large difference in dose between the toxicity studies and the clinically efficacious dose (300-1000x), it is unlikely that the administration of a booster dose will lead to significant accumulation. Finally, the applicant is of the opinion that these results support no requirements for contraception. The CHMP [which is EUrocrat-speak for ‘Committee for Medicinal Products for Human Use’, of CHMP, is the European Medicines Agency's (EMA) committee responsible for human medicines’] found this position agreeable. (p. 46)
Again, no studies of data required. The applicant’s—BioNTech/Pfizer—opinion suffices. Imagine you’d do cooking according to your opinion, which may vary from your partner’s, and you’d get the point of how insane this is.
Onwards towards the ‘Metabolism of the two novel LNP-excipients’ (pp. 47-48):
No metabolic studies were performed with the modRNA or the other two
lipids of the LNP. Overall, it seems as both ALC-0159 and ALC-0315 are metabolised by hydrolytic metabolism of the amide or ester functionalities, respectively, and this hydrolytic metabolism is observed across the species evaluated.
The metabolism of the novel excipients, ALC-0159 and ALC-0315, were examined in vitro using blood, liver S9 fractions and hepatocytes, all from mouse, rat, monkey and human. The in vivo metabolism was examined in rat plasma, urine, faeces, and liver from a rat pharmacokinetics study where a luciferase-encoding modRNA formulated in an LNP was used.
Again, no studies were done, but ‘it seems’ apparently stands in for ‘The Science’ these days.
On ‘Toxicology’ (pp. 48-51), and then I’ll stop, o.k.?
The toxicological dossier for BNT162b2 is based on a total of three pivotal toxicological experimental studies; two repeat-dose toxicity rat studies and one DART fertility-EFD rat study. The test substance in the repeat-dose toxicity studies is BNT162b2 (100 μg of variant 8 in one study (study 38166) and 30 μg of the clinically relevant variant 9 in the second study (study 20GR142)), which consists of a modified RNA in a lipid nanoparticle (LNP) formulation. The differences between the variants are due to codon optimization. The LNP contains four excipients whereof two are considered novel (ALC-0315 and ALC-0159). (p. 48)
Repeat dose toxicity (pp. 48-49):
The two general/repeat-dose toxicity studies involved IM exposure of Han Wistar rats to BNT162b2 for a total of 17 days (three weekly administrations) followed by three weeks of recovery. Overall, the study designs only included a single experimental group each with a variant of BNT162b2 (V8 or V9 variant), with no dose-response assessment or specific experimental groups for the LNP alone or its novel excipients. No test substance-linked mortality or clinical signs were observed (except a slight increase [<1C] in body temperature). No ophthalmological and auditory effects were found. The animal model of choice, the rat, has not been assessed in the pharmacological dossier but a limited absorption/ distribution study has been conducted in pharmacokinetics dossier. Immunogenicity was assessed in the toxicology studies.
I’ll skip the sections on body weight and food intake to point to Histopathology (p. 49):
At 100ug BNT162b2 V8, there were observations of various inflammatory signs at the injection site (e.g. fibrosis, myofiber degeneration, oedema, subcutis inflammation and epidermis hyperplasia). Also, there was inflammation of the perineural tissue of the sciatic nerve and surrounding bone in most rats at d17. The bone marrow demonstrated increased cellularity and the lymph nodes showed plasmacytosis, inflammation and increased cellularity. The spleen demonstrated increased haematopoiesis in half the animals at d17. The liver showed hepatocellular periportal vacuolation at d17 (fully reversed during recovery) which may be related to hepatic clearance of ALC0315. Histopathology
assessment of 30ug BNT162b2 V9 generated similar results as 100ug BNT162b2 V8 although not on as extensive level (possibly due to a lesser dose)…
The Applicant explained that peri-portal liver vacuolization was observed in both pivotal studies but are not related to any microscopic evidence of liver/biliary injury in animals (cellular hypertrophy, inflammation) nor any clinical data from Phase 1 study. Vacuoles are considered by the Applicant to be a result of ALC-0315 accumulation in liver and not PEG…
Moreover, increases in neutrophils, monocytes, eosinophils and basophils were observed in study 20GR142. For the Applicant, increases in neutrophils, monocytes, eosinophils and basophils observed in the Study 20GR142 were related to the inflammatory/immune response to BNT162b2 administration.
Similar findings were also identified in Study 38166 in animals administered 100 μg BNT162b2. The applicant stated that the increases in eosinophils and basophils are a minor component of the inflammatory leukogram, which is dominated by increases in neutrophils. The applicant also informed that characterisation of large unstained cells [keep this in mind, cf. below] was not conducted since the identification of these cells does not provide additional information. The CHMP found this agreeable.
There’s more (p. 50):
Haematology: At 30ug BNT162b2 V9 and 100ug BNT162b2 V8, there was a moderate to strong reduction of reticulocytes (48-74%, not specified for V9) coupled to lowered red cell mass parameters (RBC, HGB, and HCT). There was a moderate to strong increase (>100%) in large unclassified cells [LUC], neutrophils, eosinophils, basophils and fibrinogen that may be related to the inflammatory/immune response.
Either the CHMP can’t remember what they read two moments ago, or someone is making up shit: LUC don’t matter on p. 49, but they ‘may be related to the inflammatory/immune response’ on p. 50. How would that work, exactly?
Read that again: two doses, that’s it. Now the EMA and the EU Commission are ‘recommending’ repeat booster injections, in addition to coupling your ‘right’ to travel freely to injection uptake. What gives?
Reproduction Toxicity (pp. 50-51) similarly holds interesting stuff, but there was only a small number of rats (n = 21), which received 4 doses, it is held that
there was transient reduced body weight gain and food consumption after each dose. No effects on the estrous cycle or fertility index were observed. There was an increase (~2x) of pre-implantation loss (9.77%, compared to control 4.09%) although this was within historical control data range (5.1%-11.5%). Among foetuses (from a total of n=21 dams/litters), there was a very low incidence of gastroschisis, mouth/jaw malformations, right sided aortic arch, and cervical vertebrae abnormalities, although these findings were within historical control data… is noted that there is currently no available data on the placental transfer of BNT162b2.
Bottom Lines
No data, lots of assumptions and opinions. Also: why does the EU Commission cite a document from Feb. 2021—that relates follow-up studies to be conducted—but not, well, these studies?
There is something rotten here, and we shall explore this further in the next post.








Interesting article. It aligns with this article from Dr. Byram Bridle
https://viralimmunologist.substack.com/p/a-moratorium-on-mrna-vaccines-is?r=6sgq0&s=r&utm_campaign=post&utm_medium=email
Ema... no comments Just US servants. Unfortunately EU it's not only a joke, but mainly a system to generalize and produce one story for all. As in US. As US want us to be.
We lost all the good and ethics of Europe when EU Parliament and Commission was established. And the final shot was the euro. And as we seeing happening NATO is the arm to make it happen.
R.I.P Europe